Molecular dynamics studies on the structure and interactions of ionic liquids containing amino-acid anions
Abstract
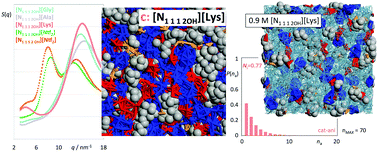
Several molecular dynamics (MD) simulations have been performed in order to obtain structural information on ionic liquids (ILs) based on amino-acid anions. Six hydrophilic ILs containing cholinium or imidazolium cations combined with alaninate, glycinate or lysinate anions were modelled using the all-atom CL&P and OPLS-AA force fields. Both pure ILs and their aqueous solutions have been studied. The MD data have allowed us to analyse structure factors, S(q), and pair radial distributions functions, g(r), as well as aggregation patterns and specific interactions. The results have shown us that in neat amino-acid-based ILs the anions interact mainly through their carboxylate moiety with the charged centres of the cations. Both the lack of heavy atoms and the small size of the interacting centre in the anion contribute to the absence of a charge ordering peak in the structure factor functions of the corresponding ILs. In turn, their aqueous solutions reveal the existence of small ionic aggregates. The size distribution of these aggregates is strongly dependent on the solution's concentration. This fact points to the possibility of using amino-acid-based ILs as agents to promote hydrotrope effects, significant for the solubilisation and stabilization of organic molecules and macromolecules in aqueous solution.


 Please wait while we load your content...
Please wait while we load your content...