Simulated and experimental force spectroscopy of lysozyme on silica†
Abstract
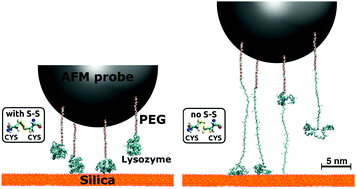
The force spectra of proteins detaching from oxide surfaces measured by atomic force microscopy (AFM) often present complex patterns of peaks, which are difficult to correlate with individual bond-breaking events at the atomic scale. In this work we rationalize experimental AFM force spectra of hen-egg-white lysozyme detaching from silica by means of all-atom steered molecular dynamics (SMD) simulations. In particular, we demonstrate that the native tertiary structure of lysozyme is preserved if, and only if, its four intramolecular disulfide bridges are intact. Otherwise, the protein pulled off the surface undergoes severe unfolding, which is well captured by SMD simulations in explicit solvent. Implicit solvent simulations, on the contrary, wrongly predict protein unfolding even in the presence of S–S bridges, due to the lack of additional structural stabilization provided by the water's hydrogen-bond network within and surrounding the protein. On the basis of our combined experimental and theoretical findings, we infer that the rugged force spectra characteristic of lysozyme/silica interfaces are not due to the successive breaking of internal disulfide bonds leading to partial unfolding events. Rather, they reflect the detachment of several molecules bound to the same AFM tip, each anchored to the surface via multiple hydrogen and ionic bonds.


 Please wait while we load your content...
Please wait while we load your content...