Evaluation of the formation and carbon dioxide capture by Li4SiO4 using in situ synchrotron powder X-ray diffraction studies†
Abstract
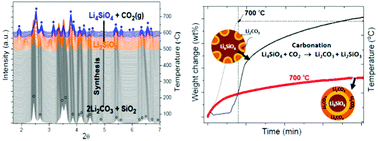
Carbon capture and storage using regenerable sorbents are an effective approach to reduce CO2 emissions from stationary sources. In this work, lithium orthosilicate (Li4SiO4) was studied as a carbon dioxide sorbent. For a deeper understanding of the synthesis and carbonation mechanism of Li4SiO4, an in situ synchrotron radiation powder X-ray diffraction technique was used. The Li4SiO4 powders were synthesized by a combination of ball milling of a Li2CO3 and SiO2 mixture followed by a thermal treatment process at low temperature. In situ studies showed that formation of Li4SiO4 from the as-milled 2Li2CO3–SiO2 mixture involves decomposition of Li2CO3 by reaction with SiO2via Li2SiO3 as an intermediate compound. No evidence of Li2Si2O5 formation was obtained, in spite of thermodynamic predictions. The CO2 capture by Li4SiO4 was evaluated dynamically over a wide temperature range, reaching a maximum weight increase of 34 wt% and good cyclability after about 10 cycles. By thermogravimetric and microstructural analyses in combination with ex situ and in situ measurements, a two step carbonation mechanism and its influence on the final CO2 capture was clearly elucidated. Under dynamical conditions up to 700 °C, the lower number of Li2CO3 nuclei initially formed retards the double shell formation and the nucleation and growth of the Li2CO3 particles remains the controlling step up to higher CO2 capture capacity. Isothermal carbonation at 700 °C favours the formation of a higher number of Li2CO3 nuclei that creates a thin carbonate shell. The CO2 diffusion through this shell is the limiting step from the beginning and further carbonation is hindered as the reaction progresses.


 Please wait while we load your content...
Please wait while we load your content...