Sampling the protonation states: the pH-dependent UV absorption spectrum of a polypeptide dyad†
Abstract
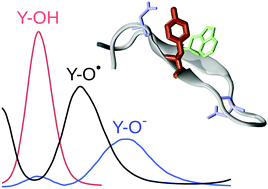
When a chromophore interacts with several titratable molecular sites, the modeling of its photophysical properties requires to take into account all their possible protonation states. We have developed a multi-scale protocol, based on constant-pH molecular dynamics simulations coupled to QM/MM excitation energy calculations, aimed at sampling both the phase space and protonation state space of a short polypeptide featuring a tyrosine–tryptophan dyad interacting with two aspartic acid residues. We show that such a protocol is accurate enough to help in the interpretation of the experimental tyrosine UV absorption spectrum at both acidic and basic pH. Moreover, it is confirmed that radical tryptophan probably contributes to the peptide spectrum, thanks to a UV-induced electron transfer from tyrosine to tryptophan, ultimately shedding light on the complex pH-dependent behavior of the peptide spectrum.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...