Resolving the halogen vs. hydrogen bonding dichotomy in solutions: intermolecular complexes of trihalomethanes with halide and pseudohalide anions†
Abstract
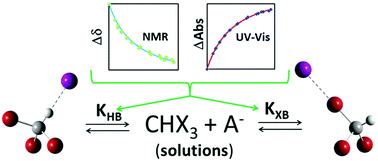
Halogen- and hydrogen-bonded complexes between trihalomethanes, CHX3, and (pseudo-)halide anions, A−, co-existing in acetonitrile solutions were identified and characterized via a combination of UV-vis and NMR spectral measurements with the results of X-ray structural and computational analyses. Halogen-bonded [CHX3, A−] complexes displayed strong absorption bands in the UV range (showing Mulliken correlations with the frontier orbital energies of the interacting species) and a decreased shift of the NMR signal of trihalomethanes' protons. Hydrogen bonding led to the opposite (increased) NMR signal shift and the UV-vis absorption bands of the hydrogen-bonded [CHX3, A−] complexes were similar in intensity to those of the separate CHX3 molecules. The simultaneous multivariable treatment of the results of UV-vis and NMR titrations of CHX3 with A− anions afforded formation constants of both halogen- and hydrogen-bonded complexes between these species, which existed side-by-side in the acetonitrile solutions. The relative values of the formation constants were consistent with the magnitudes of the positive potentials on the surfaces of the halogen or hydrogen atoms if the effects of the polarization of the trihalomethanes due to the presence of the anions were taken into account.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...
