Insights into the dissociative ionization of glycine by PEPICO experiments†
Abstract
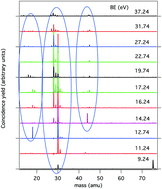
The fragmentation of glycine (NH2CH2COOH) has been studied by photoelectron–photoion coincidence, PEPICO, experiments at 60 eV photon energy. Glycine practically fragments at the ionization threshold, with the charge being on the H2NCH2+ moiety, due to ejection of an electron from the nitrogen lone pair of the highest occupied molecular orbital. To observe the formation of the complementary cation COOH+ further energy is needed. The flexibility with respect to rotation about the C–C, C–N and C–O bonds makes glycine exist in the gas phase in several conformers of both Cs and C1 point group symmetry in the neutral as well as ion states. The ionization can lead to stabilization of some conformations, rearrangements and, last but not least, H migration between the two moieties. The results of these experiments prove the sensitivity of PEPICO to pin point all these processes.


 Please wait while we load your content...
Please wait while we load your content...