Fragmentation of chlorpyrifos by thermal electron attachment: a likely relation to its metabolism and toxicity
Abstract
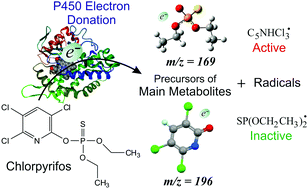
The energies of formation and dissociative decays of temporary negative ions of the organophosphorus insecticide chlorpyrifos (CPF) are studied using electron transmission spectroscopy (ETS), dissociative electron attachment spectroscopy (DEAS) and quantum-chemical calculations. Three features are displayed by ETS at 2.4, 3.1 and 4.30 eV, which are ascribed to empty σ* MOs, a higher-lying π* MO and a core-excited state, respectively. Two stable π* anion states are predicted by the calculations. Most of the negative fragments are detected by DEAS at thermal energies of the incident electrons, being thus associated with the dissociation of stable (vibrationally excited) negative ion states formed by electron attachment into the π* LUMO and LUMO+1. The CPF− molecular anions (not observed in the present study) are expected to decay by fast dissociation to give the most abundant ([CPF – HCl]−) species, which in turn dissociates on the microsecond timescale, producing as much as six metastable peaks in the mass spectrum. The m/z = 196 and 169 negative fragments, structurally similar to the main metabolites of CPF, 3,5,6-trichloro-2-pyridinol and O,O-diethyl thiophosphate, respectively, are formed by the direct decomposition of CPF−. Active radicals able to abstract hydrogen atoms from lipid membranes are generated as neutral counterparts of the observed anion fragments. A likely involvement of DEA in the biotransformation of CPF by cytochrome P450 enzymes in a reductive environment producing toxic species and precursors of the main metabolites is briefly discussed.


 Please wait while we load your content...
Please wait while we load your content...