Thermoelectrochemical cells based on Li+/Li redox couples in LiFSI glyme electrolytes†
Abstract
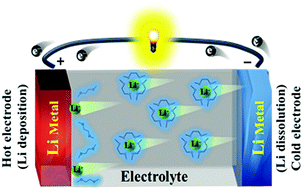
Thermoelectrochemical cells (TECs) provide conspicuous advantages, including a high Seebeck coefficient (Se), design flexibility, and low cost compared with conventional thermoelectric devices. Here, we investigated TECs employing Li metal electrodes (Li-TECs) and a series of glyme (CH3O[CH2CH2O]nCH3, n = 1–4, nG) solvents with 0.5–3.0 M lithium-imide salts (lithium bis [fluorosulfonyl]imide, LiFSI, and lithium bis[trifluoromethane sulfonyl]imide, LiTFSI). The Se value and power performance of Li-TECs markedly depend on the nature of glyme solvents and Li salt concentration. The dependency of Se on the solvation structure of the Li-ions is examined via Raman measurements, and the internal resistance of Li-TECs is analyzed using electrochemical impedance spectroscopy. Notably, a Li-TEC with 1.0 M LiFSI 1G displays about two times higher power density and about eight times higher conversion efficiency than a conventional Cu-TEC utilizing aqueous electrolytes, which is ascribed to the high Se value and low thermal conductivity of the former. In addition, for a Li-TEC with 1.0 M LiFSI 1G, the low-temperature performance is examined to assess its practical feasibility.


 Please wait while we load your content...
Please wait while we load your content...