Role of transition metals in a charge transfer mechanism and oxygen removal in Li1.17Ni0.17Mn0.5Co0.17O2: experimental and first-principles analysis†
Abstract
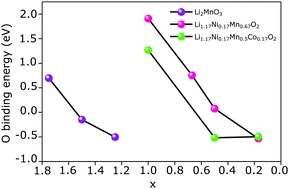
Oxygen removal from high capacity Li-rich layered oxide Li1.17Ni0.17Mn0.5Co0.17O2 affects the charge transfer process during cycling. During de-lithiation, oxygen removal takes place with the reduction in oxygen binding energy. Co substitution affects oxygen removal by shifting the O-p orbital closer to the Fermi energy. A convex hull plot is used to analyse single-phase and two-phase reactions during de-lithiation in Li1.17Ni0.17Mn0.5Co0.17O2 and Li2MnO3. Experimentally, the single-phase and two-phase reactions are identified based on the characteristics of the charge curve. In the charge transfer process more than 80% of lithium charge is transferred to oxygen in both the compounds. Effective charge and cyclic voltammetry reveal the redox centers in the compounds which help to understand the role of oxygen and transition metals in de-lithiation. A detailed explanation of oxygen removal and the charge transfer mechanism of Li1.17Ni0.17Mn0.5Co0.17O2 and Li2MnO3 is provided in the current experimental and density functional theory based study.


 Please wait while we load your content...
Please wait while we load your content...