Nonlinear optical responses of self-assembled monolayers functionalized with indolino–oxazolidine photoswitches†
Abstract
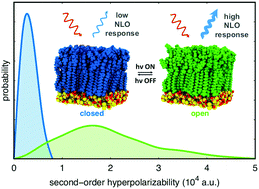
A computational approach combining molecular dynamic simulations and density functional theory (DFT) calculations is implemented to evaluate the second-order nonlinear optical (NLO) responses of photoresponsive self-assembled monolayers (SAMs) based on indolino–oxazolidine molecular switches. These numerical simulations provide a complete atomistic picture of the morphology of the SAMs, revealing a high degree of positional disorder and an almost isotropic orientation of the chromophores. Subsequent DFT calculations, carried out to evaluate the average first hyperpolarizability of indolino–oxazolidine switches within the SAM, predict that the structural disorder does not significantly reduce the NLO contrast compared to that of the isolated molecules. Chromophores in the SAM can assume a limited number of specific conformations, due to the high rotational barrier that characterize the conjugated bonds along the indolino/oxazolidine-dyene-thiophene sequence. A notable exception is the rotation about the thiophene–thioalkyl bond, which is not only almost free, but also strongly correlated with the magnitude of the first hyperpolarizability. Controlling this rotation by chemical design could thus be a viable strategy to optimize the SAMs NLO response and the performance of photoresponsive devices based on indolino/oxazolidine switches.


 Please wait while we load your content...
Please wait while we load your content...