A proton transfer network that generates deprotonated tyrosine is a key to producing reactive oxygen species in phototoxic KillerRed protein†
Abstract
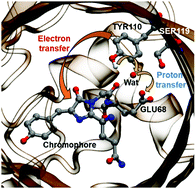
KillerRed is the first genetically encoded photosensitizer that can induce cytotoxicity upon light exposure. Nevertheless, its phototoxicity is still lower than that of chemical photosensitizers, and the efforts to further develop KillerRed variants with enhanced phototoxicity have been impeded because the mechanism by which it generates cytotoxic reactive oxygen species (ROS) has remained elusive. To shed light on this issue, we employ quantum mechanics/molecular mechanics (QM/MM) modeling with statistical free energy analysis to examine the photo-induced electron transfer reaction occurring in KillerRed. We identify a deprotonated tyrosine residue (Tyr110) as an electron donor and further show that adjacent glutamate and serine residues play essential roles in deprotonating Tyr110. We also show that water mediation is important in the proton transfer and that protein fluctuations importantly govern the fate of the excited system. We provide clues about why KillerRed can only exhibit a low ROS yield and suggest future directions of mutagenesis toward an enhanced phototoxicity.


 Please wait while we load your content...
Please wait while we load your content...