Reversible DNA compaction induced by partial intercalation of 16-Ph-16 gemini surfactants: evidence of triple helix formation†
Abstract
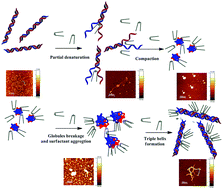
The interaction between calf thymus DNA and the gemini surfactants N,N′-[α,ω-phenylenebis(methylene)bis [N,N′-dimethyl-N-(1-hexadecyl)]-ammonium dibromide], p-16-Ph-16 (α = 1, ω = 3) and m-16-Ph-16 (α = 1, ω = 2), has been investigated via circular dichroism, fluorescence and UV-vis spectroscopy, zeta potential, dynamic light scattering, and AFM microscopy. Measurements were carried out in aqueous media at different molar ratios, R = (C16-Ph-16)/CDNA and C16-Ph-16 always below the critical micellar concentration (CMC) of the surfactant. Under these conditions, DNA undergoes two reversible conformational changes, compaction and decompaction, due to interaction with the surfactant molecules at low and high molar ratios, respectively. The extent of such conformational changes is correlated with both the degree of surfactant partial intercalation, and the size and charge of the surfactant aggregates formed, in each case. Comparison of the results shows that the para-form of the surfactant intercalates into the DNA to a major extent; therefore, the compaction/decompaction processes are more effective. Among these, the structure of the resulting 16-Ph-16/DNA decompacted complex is worthy of note. For the first time it can be demonstrated that the partial intercalation of the 16-Ph-16 gemini surfactants induces the formation of triplex DNA-like structures at a high R ratio.


 Please wait while we load your content...
Please wait while we load your content...