Understanding dissolution process of chitin crystal in ionic liquids: theoretical study†
Abstract
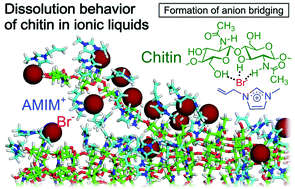
Chitin is a promising biomass resource and has high potential for industrial applications owing to its huge annual production in nature. However, it exhibits poor processability and solubility due to its very stable and crystalline character. Recently, ionic liquids (ILs) have attracted attention as solvents for structural polysaccharides – for example, 1-allyl-3-methylimidazolium bromide (AMIMBr) has been found to dissolve chitin. As few ILs are known to dissolve chitin, little research has been conducted on the dissolution mechanism involved. In this study, we have adopted a molecular dynamics (MD) approach to study the dissolution of chitin crystals in imidazolium-based ILs. The MD simulation in AMIMBr has demonstrated that the dissolution process involved peeling of chitin chains from the crystal surface, with Br− cleaving the chitin hydrogen bonds, and AMIM+ preventing a return to the crystalline phase after the peeling. By contrast, in imidazolium acetates, which has also been reported to dissolve chitin, although the molecular chains are peeled off, the peeled chains occasionally return to the crystalline phase. Furthermore, the MD trajectory analysis has revealed that the solubility of chitin is well correlated with the number of intermolecular hydrogen bonds by acetamido groups in the chitin crystal. It has been experimentally proven that mixing a small amount of 2-bromoethyl acetate, as a bromide generator, with 1-allyl-3-methylimidazolium chloride can enhance chitin solubility, which supports the dissolution mechanism indicated by the above theoretical results.


 Please wait while we load your content...
Please wait while we load your content...