Understanding the quenching nature of Mn4+ in wide band gap inorganic compounds: design principles for Mn4+ phosphors with higher efficiency†
Abstract
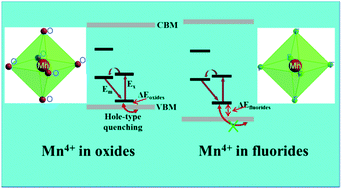
Mn4+ doped phosphors, as an alternative to rare-earth element doped phosphors, have attracted immense attention owing to their ultrahigh quantum efficiency of red emission for potential applications in high rendering white LEDs (light-emitting diodes). Their performance can be largely affected by quenching phenomena such as thermal quenching, concentration quenching and the quenching induced by some intrinsic/extrinsic defects. However, the quenching mechanisms due to the defect levels and host band are still incompletely understood. In this work, we carry out a comprehensive first-principles study on the underlying quenching mechanisms due to the defect levels of Mn4+ and other extrinsic/intrinsic defects, using the prototype oxide Y3Al5O12 (YAG), fluorides K2TiF6 (KTF) and ZnTiF6·6H2O (ZTF) as examples. From the comparison of the defect levels of Mn4+ with the host bands, we find that it is the very small energy difference between the defect levels of Mn4+ and the valence bands maximum (VBM) of YAG that causes the lower luminescence thermal stability of YAG:Mn4+, which we name as the hole-type thermal quenching mechanism. For the concentration quenching, it is nearly impossible for the Mn4+–Mn4+ pairs, previously considered as the main quenching centers, to appear in phosphors. A new quenching nature has been discussed. For the impurity ionic effects, the hole-type defects can largely stabilize the Mn ions in +4 states, thereby enhancing the emission intensity. These proposed mechanisms can offer deeper insights into the luminescence behavior of Mn4+ and a better practical understanding of the high photoluminescence quantum yield red phosphors by adjusting their chemical components.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...