Real space bond orders are energetic descriptors†
Abstract
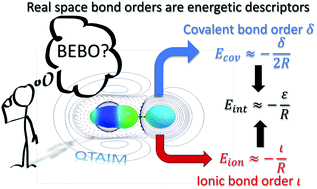
Orbital invariant position space techniques are used to show a theoretical link between the conventional concept of bond order and the energetics of chemical interactions. Taking advantage of the parallelism between the covalent and ionic interaction energies in the interacting quantum atoms (IQA) approach, a real space ionic bond order is defined. Expanding the covalent and ionic interaction energies as a multipolar series we show that the zeroth order terms in the expansion, those dominating the total interaction, are nothing but distance-scaled bond orders. A chemically intuitive picture emerges in which bonding is brought about by the Coulomb attraction of permanently transferred electrons, that give rise to ionic terms, and of the Coulombic attraction of half the shared pairs, which provide the covalent contributions. A set of representative molecules are examined to explore how the zeroth order approximation works. We show that, as expected, the approximation improves with interatomic distance.


 Please wait while we load your content...
Please wait while we load your content...