Probing hydrogen bonding interactions and impurity intercalation in gibbsite using experimental and theoretical XANES spectroscopy†
Abstract
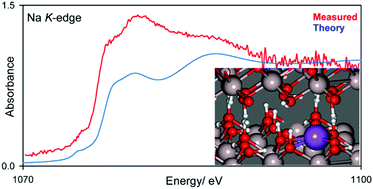
The Al and O K-edge XANES spectra of Bayer process gibbsite are examined, both experimentally and with plane wave density functional theory, and transition assignments are made. We confirm that the broad transitions in Al K-edge spectra are fundamentally the same as those in more ordered and well-studied corundum, and typical of octahedrally coordinated aluminium. Analysis of O K-edge spectra reveals that while much of the initial edge transition structure (535–548 eV) is due to O 2p hybridization with Al orbitals, the lowest energy region (∼535–539 eV) is particularly influenced by O–H states which are heavily perturbed by hydrogen bonding interactions. We therefore tentatively suggest that an unexplained structure at 539 eV in O K-edge spectra of corundum may be due to surface hydroxylation. Finally, we examined Na K-edge spectra, as sodium is the major impurity in these materials and implicated in perturbation of the evolution of materials properties with calcination through the transition aluminas. Density functional theory calculations find sodium replacement of in-sheet H-atoms is the most thermodynamically stable site for sodium inclusion. We subsequently confirm this assignment spectroscopically, explicitly demonstrating the precise location of Na+ impurities for the first time.


 Please wait while we load your content...
Please wait while we load your content...