Proton transfer in nonpolar solvents: an approach to generate electrolytes in aprotic media†
Abstract
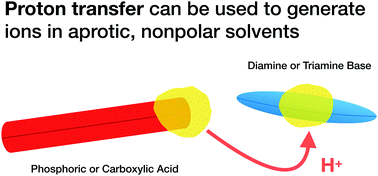
Stabilizing charged species in nonpolar solvents is challenging due to their low dielectric constant. As a contrast to formally ionic electrolytes, two series of acidic “potential” electrolytes have been developed in this study. These can be ionized by combining them stoichiometrically with a small molecule base in a typical nonpolar solvent, n-dodecane. The electrolytic conductivity of solutions of bis(2-ethylhexyl)phosphoric acid as mixtures with linear and branched dioctylamines and trioctylamines was measured, and the solutions were found to become increasingly conductive as the concentration increased, demonstrating that proton transfer occurred between the two species. Linear octylamines were found to be most effective at deprotonation. An acid-tipped poly(lauryl methacrylate) polymer (PLMA48-COOH) was also studied to give a polymer soluble in n-dodecane with a single ionizable group located precisely at the end of the polymer chain. Trioctylamine could successfully deprotonate this acid group. Even in an aprotic solvent, the transfer of protons between acidic and basic moieties is a useful method for controlling the properties of dissolved molecules.


 Please wait while we load your content...
Please wait while we load your content...