Theoretical insights into the structural and fluorescence properties of DNA containing fluorescent nucleobases†
Abstract
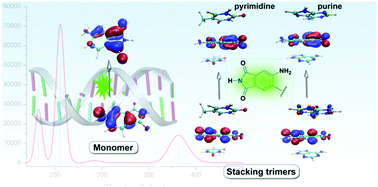
Fluorescent base analogues are of great importance as sensitive probes to detect the dynamic structures of DNA. In this research, the structural and photophysical properties of 13-mer oligonucleotides containing 4-aminophthalimide:2,4-diaminopyrimidine (4AP:DAP) (4AP0, 4AP′) were characterized using both molecular dynamics simulations and quantum mechanics methods. The results indicate that the 4AP:DAP pair is well adapted to the overall B-DNA structure with higher stability and π-stacking abilities. The structural overlap of 4AP′ and 4AP0 with the neighboring adenines only lies in the 5′-direction which results in the structure distortion from native B-DNA. Furthermore, the photophysical properties of the fluorescent base monomers and the B-DNA duplex were explored in detail. A very important result is that the hydrogen bond interaction does not have more effect on the fluorescence band apart from the slight red-shifts. In particular, the identity of the neighboring bases stacked with 4AP has an important effect on the fluorescence band. How the local environment can alter the photophysical features of the nucleobases when they are incorporated into the DNA duplex is elucidated.


 Please wait while we load your content...
Please wait while we load your content...