Time-dependent yield of the hydrated electron and the hydroxyl radical in D2O: a picosecond pulse radiolysis study†
Abstract
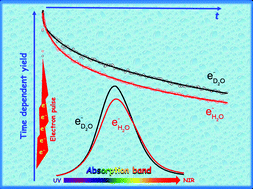
Picosecond pulse radiolysis measurements were performed in neat D2O and H2O in order to study the isotopic effect on the time-resolved yield of the hydrated electron and hydroxyl radical. First, the absorption band of the hydrated electron in D2O, eD2O−, is measured between 250 and 1500 nm. The molar absorption coefficient of the solvated electron spectrum in D2O was determined using the isosbestic point method by scavenging the solvated electron using methyl viologen. The amplitude and shape of the absorption spectrum of the hydrated electron in D2O are different from those previously reported in the literature. The maximum of the hydrated electron in the D2O absorption band is ca. 704 nm with a molar absorption coefficient of (22 900 ± 500) L mol−1 cm−1. Based on this extinction coefficient, the radiolytic yield of eD2O− just after the 7 ps electron pulse was determined to be (4.4 ± 0.2) × 10−7 mol J−1, which coincides with the one for eH2O− in H2O. The time-dependent radiolytic yield of eD2O− was determined from a few ps to 8 ns. To determine the OD˙ radical yield, the contribution of the solvated electron and of the transient species produced by the electron pulse in the windows of the fused silica optical cell was taken into account for the analysis of the transient absorption measurements at 260 nm. Therefore, an appropriate experimental methodology is used for measuring low absorbance at two different wavelengths in ps pulse radiolysis. The yield of the OD˙ radical just after the 7 ps electron pulse was found to be (5.0 ± 0.2) × 10−7 mol J−1. In the spurs of ionization, the decay rate of eD2O− is slower than eH2O−, whereas the decay rate of OD˙ is similar to the one of OH˙. Here, the established time-dependent yield of the solvated electron and the hydroxyl radical provide the foundation for improving the models used for spur reaction simulations in heavy water mainly for the chemistry of CANDU reactors.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...