How low-resolution structural data predict the conformational changes of a protein: a study on data-driven molecular dynamics simulations†
Abstract
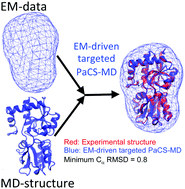
Parallel cascade selection molecular dynamics (PaCS-MD) is a conformational sampling method for generating transition pathways between a given reactant and a product. PaCS-MD repeats the following two steps: (1) selections of initial structures relevant to transitions and (2) their conformational resampling. When selecting the initial structures, several measures are utilized to identify their potential to undergo transitions. In the present study, low-resolution structural data obtained from small angle scattering (SAXS) and cryo-electron microscopy (EM) are adopted as the measures in PaCS-MD to promote the conformational transitions of proteins, which is defined as SAXS-/EM-driven targeted PaCS-MD. By selecting the essential structures that have high correlations with the low-resolution structural data, the SAXS-/EM-driven targeted PaCS-MD identifies a set of transition pathways between the reactant and the product. As a demonstration, the present method successfully predicted the open–closed transition pathway of the lysine-, arginine-, ornithine-binding protein with a ns-order simulation time, indicating that the data-driven PaCS-MD simulation might work to promote the conformational transitions of proteins efficiently.


 Please wait while we load your content...
Please wait while we load your content...