The predictive power of aromaticity: quantitative correlation between aromaticity and ionization potentials and HOMO–LUMO gaps in oligomers of benzene, pyrrole, furan, and thiophene†
Abstract
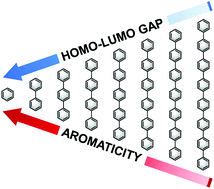
Aromaticity is a central and ubiquitous concept in organic chemistry, and is used extensively to explain various phenomena. Yet, aromaticity cannot be observed or measured as a property in its own right and, to date, only qualitative and semi-quantitative relationships have been described between aromaticity and an observable property. We now demonstrate for the first time a robust quantitative relationship between the HOMO–LUMO gap and adiabatic ionization potential of a polycyclic aromatic hydrocarbon oligomer – both measurable physical quantities – and its aromaticity, as quantified by the Nucleus Independent Chemical Shift (NICS) index. The agreement found for a range of structurally and electronically diverse oligomeric systems of varying lengths is so well-behaved as to enable accurate prediction of the properties of longer members of the respective oligomer family. The established correlation allows for preliminary screening of compounds geared towards functional use.


 Please wait while we load your content...
Please wait while we load your content...