Effects of surface hydroxylation on adhesion at zinc/silica interfaces
Abstract
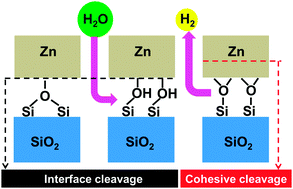
The weak interaction between zinc and silica is responsible for the poor performance of anti-corrosive galvanic zinc coatings on modern advanced high-strength steels, which are fundamental in the automotive industry, and important for rail transport, shipbuilding, and aerospace. With the goal of identifying possible methods for its improvement, we report an ab initio study of the effect of surface hydroxylation on the adhesion characteristics of model zinc/β-cristobalite interfaces, representative of various surface hydroxylation/hydrogenation conditions. We show that surface silanols resulting from dissociative water adsorption at the most stable stoichiometric (001) and (111) surfaces prevent strong zinc–silica interactions. However, dehydrogenation of such interfaces produces oxygen-rich zinc/silica contacts with excellent adhesion characteristics. These are due to partial zinc oxidation and the formation of strong iono-covalent Zn–O bonds between zinc atoms and the under-coordinated excess anions, remnant of the hydroxylation layer. Interestingly, these interfaces appear as the most thermodynamically stable in a wide range of realistic oxygen-rich and hydrogen-lean environments. We also point out that the partial oxidation of zinc atoms in direct contact with the oxide substrate may somewhat weaken the cohesion in the zinc deposit itself. This fundamental analysis of the microscopic mechanisms responsible for the improved zinc wetting on pre-hydroxylated silica substrates provides useful guidelines towards practical attempts to improve adhesion.


 Please wait while we load your content...
Please wait while we load your content...