Effect of unsaturated substituents in the reaction of Criegee intermediates with water vapor†
Abstract
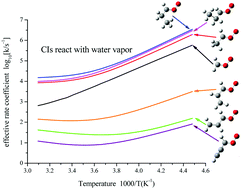
Criegee intermediates (CIs), formed in the reactions of unsaturated hydrocarbons with ozone, are very reactive carbonyl oxides and have recently been suggested as important oxidants in the atmosphere. In this work, we studied the substituent effect on the water monomer and dimer reaction with CIs which include up to three carbon atoms at the QCISD(T)/CBS//B3LYP/6-311+G(2d,2p) level. Our calculation showed that for saturated CIs with a hydrogen atom on the same side as the terminal oxygen atom, the reaction with water vapor would likely dominate the removal processes of these CIs in the atmosphere. On the other hand, for unsaturated CIs, the reactivity toward water vapor decreases compared to the saturated species allowing them to survive in humid atmospheric environments. We also evaluated the kinetic isotope effect in the reaction between CI and water vapor by performing calculations with deuterated water. We found that tunneling is not important and the kinetic isotope effect mainly comes from the difference in the zero point energy between water and deuterated water.


 Please wait while we load your content...
Please wait while we load your content...