The role of hydrophobic hydration in the LCST behaviour of POEGMA300 by all-atom molecular dynamics simulations†
Abstract
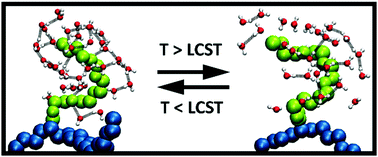
The solubility and lower critical solution temperature (LCST) behaviour of poly(oligo(ethylene glycol)methyl ether methacrylate) (POEGMA300) in water were comprehensively investigated by all-atom molecular dynamics (MD) simulations for 5-, 20-, 50- and 75-mer homopolymers. According to various structural and dynamic properties, the water-solubility of POEGMA300 below the LCST is mainly provided by hydrophobic hydration around the side chain carbon atoms, which is achieved by cage-like water formations. The LCST phase transition occurs when these cage-like structures are disrupted by increasing the temperature above the LCST. During this process, significant amounts of water molecules are released and the local water-ordering is reduced. Moreover, the number of hydrogen bonds and hydrogen bond lifetime results indicate that the hydrogen bonding between polymers and water molecules has relatively little effect on the phase transition. Also, the diffusion rates of 50- and 75-mer POEGMA300 decrease with increasing temperature, which may be due to the breakage of cage-like water structures when the polymer exceeds a certain chain length. Our atomistic level findings will enhance the understanding of the LCST phase transition of OEGMA based homopolymers and will be helpful to design homo- and co-polymers of OEGMAs with required properties.


 Please wait while we load your content...
Please wait while we load your content...