A kinetics study of the heterogeneous reaction of n-butylamine with succinic acid using an ATR-FTIR flow reactor
Abstract
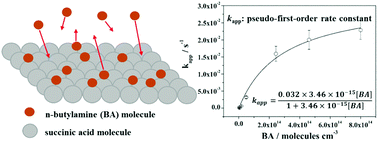
Despite increased awareness of the roles played by atmospheric amines and carboxylic acids in an atmospheric environment, current knowledge of heterogeneous reactions between atmospheric amines and carboxylic acids, especially how the reaction rates vary with different ambient conditions including temperature, relative humidity (RH), and reactant concentration, remains very scarce. Here, the kinetics of the heterogeneous reaction of n-butylamine (BA) with succinic acid (SA) were studied via a flow system combined with attenuated total reflection Fourier-transformed infrared spectroscopy (ATR-FTIR) over a wide range of temperatures (263–295 K), BA concentrations (0.145–32.5 ppm), thin film thicknesses (0.05–0.15 μm), and RHs (0–75%) under atmospheric pressure conditions for the first time. Pseudo-first-order rate constant (kapp) and overall reactive uptake coefficient (γ) values were derived according to the changes in absorbance from the peak located near 1634 cm−1, which can be assigned to the –COO− antisymmetric stretch (νas(–COO−)). The results show that both the kapp and γ values display very strong temperature dependence, and low temperatures promote the reaction. According to the kapp values as a function of temperature, the activation energy for the heterogeneous reaction is estimated to be −71.9 kJ mol−1. Such a phenomenon indicates that physisorption of BA at the surface of the SA thin film is probably the rate-limiting step for the overall reaction. Meanwhile, the heterogeneous reaction of SA with BA follows the Langmuir–Hinshelwood mechanism, which confirms that kapp is largely dominated by the surface reaction over the bulk phase reaction. In addition, with increasing RH, both the kapp and γ values increase considerably, indicating that the presence of water vapor has a synergistic effect on the reaction. Water uptake results also show that the hygroscopic behavior of the thin film is greatly enhanced after BA exposure.


 Please wait while we load your content...
Please wait while we load your content...