Shape transition of water-in-CO2 reverse micelles controlled by the surfactant midpiece†
Abstract
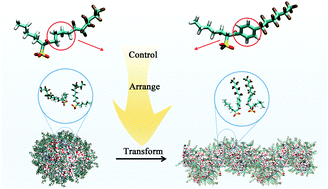
Designing CO2-philic surfactants for generating wormlike reverse micelles (RMs) is an effective approach to enhance the viscosity of supercritical CO2 (scCO2), however this remains challenging. Modifying the middle piece (midpiece) of surfactant tails is a potential method to generate wormlike RMs, but the underlying mechanism is still unclear. Herein, by adopting molecular dynamics simulations, the self-assembly of the hybrid surfactant FC6–HC5 in scCO2 was investigated. It was found that the FC6–HC5 with an alkyl midpiece could form spherical RMs. By introducing phenyl on the surfactant midpiece, a transformation of the RMs from a spherical shape to a wormlike shape was achieved. The improved fusion free energy was demonstrated to promote the fusion of the spherical RMs to form wormlike RMs. Further analysis indicated that, originating from the π–π interaction, the introduced phenyl assists the parallel arrangement of FC6–HC5, resulting in the improved fusion ability. Moreover, according to the analysis on interfacial properties, introducing phenyl had little effect on the surfactant CO2-philicity. Therefore, modifying the midpiece is a great method for designing hybrid surfactants to generate wormlike RMs while maintaining their high CO2-philicity. This strategy of generating wormlike RMs is expected to facilitate the application of scCO2 meeting industrial requirements.


 Please wait while we load your content...
Please wait while we load your content...