Mobility of hydrous species in amorphous calcium/magnesium carbonates†
Abstract
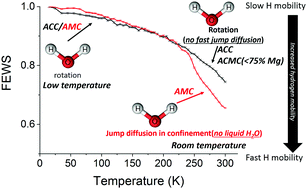
Amorphous calcium carbonate (ACC) is commonly found in many biological materials. As ACC readily crystallizes into calcite, stabilizers, such as anions, cations or macromolecules, often occur to avoid or delay unwanted crystallization. In biogenic ACC, magnesium is commonly present as one of the stabilizing agents. It is generally thought that the presence of mobile water in ACC is responsible for its limited stability and that the strong interaction of Mg2+ with water stabilizes the amorphous structure by retarding dehydration of ACC. To test this hypothesis, we studied the mobility of hydrous species in the model materials ACC, amorphous magnesium carbonate (AMC) and amorphous calcium/magnesium carbonate (ACMC), using quasi elastic neutron scattering (QENS) which is highly sensitive to the dynamics of H atoms. We discovered that hydrous species in the considered amorphous materials consist of water and hydroxide ions, as magnesium ions are incorporated in a ratio of 1 to about 0.6 with OH−. Surprisingly, we found that there is no evidence of translational diffusion of water and hydroxides when calcium is present in the samples, showing that hydrous species are highly static. However, we did observe diffusion of water in AMC with similar dynamics to that found for water in clays. Our results suggest that Mg2+–water interactions alone are not the only reason for the high stability of AMC and ACMC. The stabilizing effect of Mg ions, in addition to Mg–water binding, is likely to be caused by binding to hydroxide in amorphous calcium carbonates. In fact, the incorporation of hydroxides into the amorphous phase results in a mineral composition that is incompatible with any of the known Ca/Mg-carbonate crystal phases, requiring large scale phase separation to reach the composition of even the basic magnesium carbonate minerals artinite and hydromagnesite.


 Please wait while we load your content...
Please wait while we load your content...