Mechanistic insights into the photogeneration and quenching of guanine radical cation via one-electron oxidation of G-quadruplex DNA†
Abstract
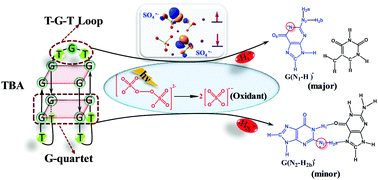
Herein, mechanistic aspects of the photogeneration and quenching of guanine radical cation through one-electron oxidation of the G-quadruplex of G2T2G2TGTG2T2G2 (TBA) sequence were investigated by a combined quantum mechanical/molecular mechanical (QM/MM) approach at the CASPT2//CASSCF/AMBER level of theory. Herein, one electron promotion of the oxygen lone pair of the photo-excited photosensitizer peroxydisulfate to its O–O σ* orbital was first demonstrated to become tunable through the varied reduction ability of the G base in the presence or absence of interbase hydrogen bonding, thereby dynamically controlling the deprotonation site in G-quadruplex TBA. The quenching of G radical cation mediated by the formation of SO42−via photoinduced electron transfer can be triggered effectively by the deprotonation reaction of free proton rather than that of the hydrogen-bonded proton in G–G (G-quartet) and G–T (loop) aqueous surrounding. By calculating the deprotonation paths for the G radical cation, the deprotonation reactions in G-quadruplex TBA were verified to proceed predominantly along the site of imino proton (N1–H) in the loop moiety; this showed the coexisting occurrence of amino (N2–H) deprotonation in the G-quartet part. The mechanistic features discussed in this study represent significant advances in the understanding of DNA radical chemistry.


 Please wait while we load your content...
Please wait while we load your content...