What atomic properties of metal oxide control the reaction threshold of solid elemental fuels?†
Abstract
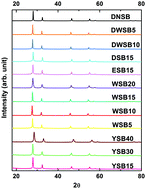
The redox reaction between fuel (metal, metalloid, etc.) and metal oxide is ubiquitous. On the other hand simple thermodynamic considerations do not seem to yield much insight into what makes for a vigorous oxidizer. In this study, two different systematically doped metal oxide systems: perovskites (9 compounds) and δ-Bi2O3 (12 compounds) were synthesized in a manner such that for each system the crystal structure and morphology were maintained. Four fuels (Al, B, Ta, C) with different physical properties, covering almost all fuel types, were included in this study. The initiation temperature and oxygen release temperature was measured by fast heating (>105 K s−1) temperature-jump/time-of-flight mass spectrometry coupled with high-speed imaging. These results were then correlated with the average metal–oxygen bond energy in the oxidizer, and overall metal–oxygen electronegativity. In general, within each systematic metal oxide, we found linear relationships between average bond energy and electronegativity of the metal oxides with initiation temperature for all four fuels, despite their very different physical/chemical properties. These results indicate that intrinsic atomic properties of metal oxide control fuel-metal oxide reaction initiation.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...
