Nitroxide–nitroxide and nitroxide–metal distance measurements in transition metal complexes with two or three paramagnetic centres give access to thermodynamic and kinetic stabilities†‡
Abstract
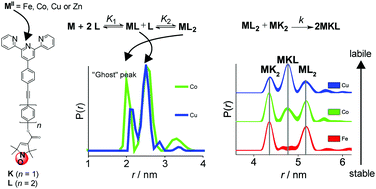
Fundamentally, the stability of coordination complexes and of templated (bio)macromolecular assemblies depends on the thermodynamic and kinetic properties of the intermediates and final complexes formed. Here, we used pulse EPR (electron paramagnetic resonance) spectroscopy to determine the stabilities of nanoscopic assemblies formed between one or two nitroxide spin-labelled tridentate 2,2′:6′,2′′-terpyridine (tpy) ligands and divalent metal ions (FeII, ZnII, CoII and CuII). In three distinct approaches we exploited (a) the modulation depth of pulsed electron–electron double resonance (PELDOR) experiments in samples with increasing metal-to-ligand ratios, (b) the frequencies of PELDOR under broadband excitation using shaped pulses and (c) the distances recovered from well-resolved PELDOR data in fully deuterated solvents measured at 34 GHz. The results demonstrate that PELDOR is highly sensitive to resolving the stability of templated dimers and allows to readily distinguish anti-cooperative binding (for CuII ions) from cooperative binding (for CoII or FeII ions). In the case of paramagnetic ions (CoII and CuII) the use of broadband PELDOR allowed to identify the cooperativity of binding from the time domain and distance data. By using a second labelled tpy ligand and by mixing two homoleptic complexes of the same metal centre we could probe the kinetic stability on a timescale of tens of seconds. Here, tpy complexes of CuII and ZnII were found to be substitutionally labile, CoII showed very slow exchange and FeII was inert under our conditions. Not only do our chemical models allow studying metal–ligand interactions via PELDOR spectroscopy, the design of our study is directly transferable to (bio)macromolecular systems for determining the kinetic and thermodynamic stabilities underpinning (templated) multimerisation. Considering the limited methods available to obtain direct information on the composition and stability of complex assemblies we believe our approach to be a valuable addition to the armoury of methods currently used to study these systems.


 Please wait while we load your content...
Please wait while we load your content...