Sub-nanometer pore formation in single-molecule-thick polyurea molecular-sieving membrane: a computational study†
Abstract
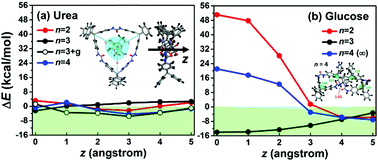
A polymeric network of 1-(4-tritylphenyl)urea (TPU) built via layer-by-layer cross-linking polymerization has been proposed to be an excellent mesh equipped with single-molecule-thick pores (i.e., cyclic poly-TPU rings), which can sieve glucose (∼0.7 nm) out of its mixture with urea for hemodialysis applications. Monte Carlo search for the lowest-energy conformation of various sizes of poly-TPU rings unravels the origin of narrow pore size distribution, which is around the sizes of dimer and trimer rings (0.3–0.8 nm). Flexible rings larger than the dimer and trimer rings, in particular tetramer rings, prefer a twisted conformation in the shape of the infinity symbol (∞, which looks like two dimer rings joined together) locked by a hydrogen bond between diphenylurea linker groups facing each other. Translocation energy profiles across these TPU rings reveal their urea-versus-glucose sieving mechanism: glucose is either too large (to enter dimers and twisted tetramers) or too perfectly fit (to exit trimers), leaving only a dimer-sized free space in the ring, whereas smaller-sized urea and water pass through these effective dimer-sized rings (bare dimers, twisted tetramers, and glucose-filled trimers) without encountering a substantial energy barrier or trap.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...