Revealing the formation and electrochemical properties of bis(trifluoromethanesulfonyl)imide intercalated graphite with first-principles calculations†
Abstract
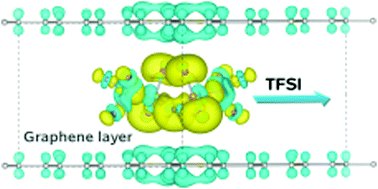
Graphite has been reported to have anion, as well as cation, intercalation capacities as both a cathode and an anode host material for dual ion batteries. In this work, we study the intercalation of bis(trifluoromethanesulfonyl)imide (TFSI) anions from an ionic liquid electrolyte into graphite with first-principles calculations. We build models for TFSI–Cn compounds with systematically increasing graphene sheet unit cell sizes and investigate their stabilities by calculating the formation energy, resulting in the linear decrease of and arrival at the limit of stability. With unit cell sizes identified for stable compound formation, we reveal that the interlayer distance and relative volume expansion ratio of TFSI–Cn increases as we increase the concentration of the TFSI intercalate during the charge process. The electrode voltage is determined to range from 3.8 V to 3.0 V at a specific capacity ranging from 30 mA h g−1 to 54 mA h g−1, in agreement with experiment. Moreover, a very low activation barrier of under 50 meV for TFSI migration, as well as a good electronic conductivity, provide evidence for using these compounds as a promising cathode. Through the analysis of the charge transfer, we clarify the mechanism of TFSI–Cn formation, and reveal new prospects for developing graphite based cathodes.


 Please wait while we load your content...
Please wait while we load your content...