Single-layer ZnMN2 (M = Si, Ge, Sn) zinc nitrides as promising photocatalysts†
Abstract
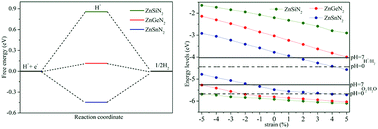
Searching for two-dimensional semiconductor materials that are suitable for visible-light photocatalytic water splitting provides a sustainable solution to deal with the future energy crisis and environmental problems. Herein, based on first-principles calculations, single-layer ZnMN2 (M = Si, Ge, Sn) zinc nitrides are proposed as efficient photocatalysts for water splitting. Stability analyses show that the single-layer ZnMN2 zinc nitrides exhibit energetic and dynamical stability. The electronic properties reveal that all of the single-layer ZnMN2 zinc nitrides are semiconductors. Interestingly, single-layer ZnSnN2 is a direct band gap semiconductor with a desirable band gap (1.74 eV), and the optical adsorption spectrum confirms its optical absorption in the visible light region. The hydrogen evolution reaction (HER) calculations show that the catalytic activity for single-layer ZnMN2 (M = Ge, Sn) is better than that of single-layer ZnSiN2. Furthermore, the band gaps and band edge positions for the single-layer ZnMN2 zinc nitrides can be effectively tuned by biaxial strain. Especially, single-layer ZnGeN2 can be effectively tuned to match better with the redox potentials of water and enhance the light absorption in the visible light region at a tensile strain of 5%, which is confirmed by the corresponding optical absorption spectrum. Our results provide guidance for experimental synthesis efforts and future searches for single-layer materials suitable for photocatalytic water splitting.


 Please wait while we load your content...
Please wait while we load your content...