Understanding the function of water during the gelation of globular proteins by temperature-dependent near infrared spectroscopy
Abstract
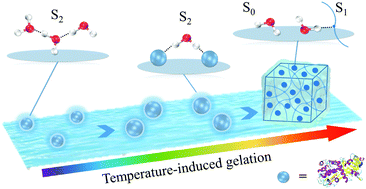
Water plays an indispensable role in the gelation of proteins, but its function still remains unclear. In this work, the variation of water species with the structural changes of globular proteins was investigated using temperature-dependent near infrared (NIR) spectroscopy. Ovalbumin (OVA) was used as a model protein, which forms a gel-like structure as the temperature increases through three phases, i.e., phase I (native), phase II (molten globule state), and phase III (gel state). The structural change and the content variation of different water species in the three phases of gelation were analyzed by two-dimensional correlation NIR spectroscopy and Gaussian fitting. A decrease in the water species with two hydrogen bonds (S2) was found and the change follows the same phases as OVA. In the first two phases, the change occurs after those of other water species but in the third phase, the change is faster than that of free water species. The result indicates that in the native and molten globule states, S2 is located in the hydration shell of OVA to maintain the stability of the protein structure, and then in the gel state, high temperature weakens the hydrogen bonding of S2 and leads to the destruction of the hydration shell, making OVA clusters form a gel structure.


 Please wait while we load your content...
Please wait while we load your content...