Hydrogen bond basicity of ionic liquids and molar entropy of hydration of salts as major descriptors in the formation of aqueous biphasic systems†
Abstract
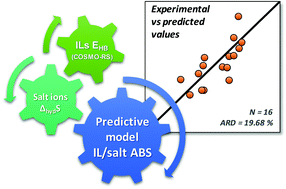
Aqueous biphasic systems (ABS) composed of ionic liquids (ILs) and conventional salts have been largely investigated and successfully used in separation processes, for which the determination of the corresponding ternary phase diagrams is a prerequisite. However, due the large number of ILs that can be prepared and their high structural versatility, it is impossible to experimentally cover and characterize all possible combinations of ILs and salts that may form ABS. The development of tools for the prediction and design of IL-based ABS is thus a crucial requirement. Based on a large compilation of experimental data, a correlation describing the formation of IL-based ABS is shown here, based on the hydrogen-bonding interaction energies of ILs (EHB) obtained by the COnductor-like Screening MOdel for Real Solvents (COSMO-RS) and the molar entropy of hydration of the salt ions. The ability of the proposed model to predict the formation of novel IL-based ABS is further ascertained.


 Please wait while we load your content...
Please wait while we load your content...