Alloy-composition-dependent oxygen reduction reaction activity and electrochemical stability of Pt-based bimetallic systems: a model electrocatalyst study of Pt/PtxNi100−x(111)
Abstract
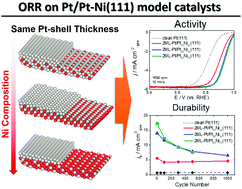
The oxygen reduction reaction (ORR) activity and electrochemical stability of well-defined n monolayer (ML)-Pt/PtxNi100−x(111) (n = 2 and 4; x = 75, 50, and 25) model electrocatalyst surfaces were investigated in this study. The initial activity of the as-prepared two-monolayered Pt-covered PtxNi100−x(111) substrates (2ML-Pt/PtxNi100−x(111)) increased with increasing Ni composition in the PtxNi100−x(111) substrate. In particular, 2ML-Pt/Pt25Ni75(111) showed the initial activity that was 25 times higher than that of clean Pt(111) although the higher Ni composition resulted in destabilization of the catalyst upon the application of potential cycles (PCs). As for 4ML-Pt/PtxNi100−x(111), activity enhancements were insensitive to alloy composition and thicker Pt shell layers stabilized the catalyst against PC applications. In particular, the activities of 4ML-Pt/Pt50Ni50(111) and 4ML-Pt/Pt25Ni75(111) gradually increased during 1000 PCs probably because of the PC-induced mono-atomic heights and nanometer-size islands that had (110) and (100) steps introduced into the topmost (111) terraces. Thus, the simultaneous tuning of core–alloy composition and Pt shell thickness is vital for developing practical, highly efficient Pt-based alloy ORR electrocatalysts.


 Please wait while we load your content...
Please wait while we load your content...