Charge-induced structural transition between seashell-like B29− and B29+ in 18 π-electron configurations†
Abstract
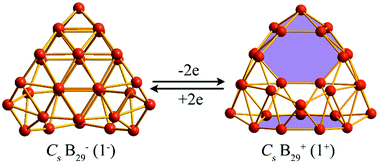
Recent joint experimental and theoretical investigations have shown that seashell-like C2 B28 is the smallest neutral borospherene reported to date, while seashell-like Cs B29− (1−) as a minor isomer competes with its quasi-planar counterparts in B29− cluster beams. Extensive global minimum searches and first-principles theory calculations performed in this work indicate that with two valence electrons detached from B29−, the B29+ monocation favors a seashell-like Cs B29+ (1+) much different from Cs B29− (1−) in geometry which is overwhelmingly the global minimum of the system with three B7 heptagonal holes in the front, on the back, and at the bottom, respectively, unveiling an interesting charge-induced structural transition from Cs B29− (1−) to Cs B29+ (1+). Detailed bonding analyses show that with one less σ bond than B29− (1−), Cs B29+ (1+) also possesses nine delocalized π-bonds over its σ-skeleton on the cage surface with a σ + π double delocalization bonding pattern and follows the 2(n + 1)2 electron counting rule for 3D spherical aromaticity (n = 2). B29+ (1+) is therefore the smallest borospherene monocation reported to date which is π-isovalent with the smallest neutral borospherene C2 B28. The IR, Raman, and UV-vis spectra of B29+ (1+) are computationally simulated to facilitate its spectroscopic characterization.


 Please wait while we load your content...
Please wait while we load your content...