A unified model of Grignard reagent formation†
Abstract
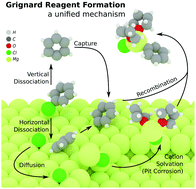
Grignard reagents are among the most fundamental reagents in organic synthesis, yet studies have hitherto failed to fully explain the selectivity and kinetics of Grignard reagent formation (GRF). The present study provides new insights into the intermediates and pathways of GRF using density functional theory (DFT) calculations. Potential energy surfaces of RX dissociation along different directions reveal the origin of configuration retention of alkenyl and aromatic halides. Radical intermediates participate solely in the dissociation stage, and depend on the geometry of the reactant halide. Dissociation of organic halides yields stabilized surface anions, and the rest of the reaction is ionic in nature. MgX+/RMg+ were proposed as the key intermediates of Mg leaving from the surface in the self-activation of GRF, which explains the accelerated kinetics upon addition of RMgX or MgX2. The intermediacy of the cations was supported by a simple electrochemical experiment. To the best of our knowledge, this is the first unified ionic model (I-model) developed for resolving the controversial issues of GRF.


 Please wait while we load your content...
Please wait while we load your content...