Adsorption of alcohols and hydrocarbons on nonstoichiometric cementite{010} surfaces†
Abstract
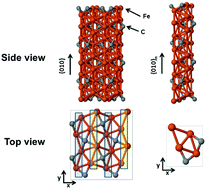
We investigate the adsorption of several organic molecules on a nonstoichiometric {010} surface of Fe3C (cementite) by means of density functional theory calculations with van der Waals corrections. The molecules studied include methanol, ethanol, n-heptane, isooctane, benzene, toluene, cyclohexane, naphthalene, 1-methylnaphthalene and decalin. We find that methanol and ethanol chemisorb over the surface, with adsorption heats between 1.0 and 1.2 eV, through their OH groups. In contrast, n-heptane, isooctane and decalin physisorb over the surface, preferentially in a flat configuration, with adsorption heats between 0.19 and 0.27 eV per carbon atom in the molecule. Aromatic molecules strongly chemisorb over the surface of cementite in a flat configuration with adsorption heat of ∼0.31 eV per molecular carbon atom. Increase of the coverage reduces the adsorption heat in all the cases considered. Dehydrogenation is disfavoured in the adsorbed nonaromatic molecules, and neutral or slightly exothermic in the aromatic ones. The adsorption process induces a small spin polarization in the rings of adsorbed aromatic molecules. The relatively strong adsorption of all the molecules considered makes them possible candidates for nucleation processes on the cementite surface.


 Please wait while we load your content...
Please wait while we load your content...