Fluorine substituent effect on the stereochemistry of catalyzed and non-catalyzed Diels–Alder reactions. The case of R-butenone with cyclopentadiene: a computational assessment of the mechanism†
Abstract
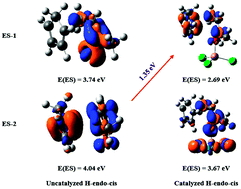
The present work studies theoretically the mechanisms involved in the fluorine substituent effect on the stereochemistry of Diels–Alder reactions. The case of R-butenone with cyclopentadiene is used for the purpose of modelling more general α-fluoro-α,β-unsaturated carbonyl compounds, in catalyzed and uncatalyzed cases. A thorough analysis of the mechanism is performed using energy decomposition analysis (EDA) and conceptual DFT tools. It is shown that the endo conformation is privileged in all the studied cases with the exception of the α-fluorinated ketone. It is found that the endo selectivity of the non-fluorinated reactions is only due to the decrease of dispersion energy. On the other hand, the presence of a fluorine atom in the dienophile moieties increases remarkably the magnitude not only of the interaction energy between the reactants but that of the strain energy as well. Moreover, it is the strong destabilization strain energy occurring at the transition state of the endo pathway of the reaction cyclopentadiene/3-fluorobutenone that is mainly responsible for the exo selectivity. The effect of a Lewis acid catalyst on these reactions is also studied. The Lewis acid catalyst affects the activation energy of the studied Diels–Alder reactions but not their stereoselectivity. Furthermore, the dual descriptor results shed light onto the mechanism. Besides, natural bond orbital analysis (NBO) and determination of the condensed values of the state-specific dual descriptors (SSDD) are carried out to evaluate the donor–acceptor properties in these reactions. For the first time, a semiquantitative prediction of stereoselectivity due to substitutions of dienophile is obtained, thus complementing the previous interpretations (R. Hoffmann and R. B. Woodward, J. Am. Chem. Soc., 1965, 87, 4388; I. Fernández and F. M. Bickelhaupt, Chem. Soc. Rev., 2014, 43, 4953). Finally, since the role of dispersion forces is evidenced in some cases, a comparison between some popular exchange–correlation functionals is presented, assessing the performance of some standard functionals besides functionals with explicit dispersion corrections.


 Please wait while we load your content...
Please wait while we load your content...