Hydrogen adsorption trends on Al-doped Ni2P surfaces for optimal catalyst design
Abstract
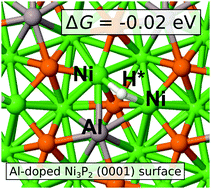
Nanoparticles of nickel phosphide are promising materials to replace the currently used rare Pt-group metals at cathode-side electrodes in devices for electrochemical hydrogen production. Chemical modification by doping can be used to fine-tune the electrocatalytic activity, but this path requires theoretical, atomic-level support which has not been widely available for Ni–P. We present a density functional theory analysis of Al-doped Ni2P surfaces to identify structural motifs that could contribute to the improved behavior of the catalyst. Based on the formation energies of substitutionally Al-doped Ni sublattices, we find doping to take place preferably at the topmost layers. The Ni–Ni bridge and the P-top sites are the optimal ones in terms of hydrogen bonding energies. The Ni–Ni bridge site is not present on pristine surfaces but is a consequence of Al doping and provides a candidate to explain the experimentally observed high activities in doped Ni–P nanoparticles. Similar structural motifs can be recommended to be engineered for other Ni–P structures for improved electrocatalytic activity.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...