Revisiting the catalytic mechanism of Mo–Cu carbon monoxide dehydrogenase using QM/MM and DFT calculations†
Abstract
Previous density functional theory (DFT) studies have shown that the release of the produced carbon dioxide (CO2) from an active-site cluster is a thermodynamically or kinetically difficult step in the enzymatic carbon monoxide (CO) oxidation catalyzed by Mo–Cu carbon monoxide dehydrogenase (Mo–Cu CODH). To better understand the effect of the protein environment on this difficult CO2 release step as well as other reaction steps, we applied hybrid quantum mechanics and molecular mechanics (QM/MM) calculations to the Mo–Cu CODH enzyme. The results show that in the first step, the equatorial Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group in the active-site cluster attacks the nearby CO molecule bound to the Cu site. Afterward, a stable thiocarbonate intermediate is formed in which the CO2 molecule is embedded and the copper–S(μ-sulfido) bond is broken. A free CO2 molecule, i.e., the final product, is then released from the active-site cluster, not directly from the thiocarbonate intermediate but via a previously formed intermediate that also contains CO2 but retains the Cu–S(μ-sulfido) bond. In contrast to the previous DFT results, the calculated barrier for this process was low in our QM/MM calculations. An additional QM/MM analysis of the barrier height showed that the effect of the protein environment on this barrier lowering is not very large. We found that the reason for the low barrier obtained by QM/MM is that the barrier for CO2 release is already not high at the DFT level. These results allow us to conclude that the CO oxidation reaction passes through the formation of a thiocarbonate intermediate, and that the subsequent CO2 release is kinetically not difficult. Nevertheless, the protein environment has an important role to play in making the latter process thermodynamically favored. No low-barrier pathway for the product release could be obtained for the reaction of n-butylisocyanide, which is consistent with the experimental fact that n-butylisocyanide inhibits Mo–Cu CODH.
O group in the active-site cluster attacks the nearby CO molecule bound to the Cu site. Afterward, a stable thiocarbonate intermediate is formed in which the CO2 molecule is embedded and the copper–S(μ-sulfido) bond is broken. A free CO2 molecule, i.e., the final product, is then released from the active-site cluster, not directly from the thiocarbonate intermediate but via a previously formed intermediate that also contains CO2 but retains the Cu–S(μ-sulfido) bond. In contrast to the previous DFT results, the calculated barrier for this process was low in our QM/MM calculations. An additional QM/MM analysis of the barrier height showed that the effect of the protein environment on this barrier lowering is not very large. We found that the reason for the low barrier obtained by QM/MM is that the barrier for CO2 release is already not high at the DFT level. These results allow us to conclude that the CO oxidation reaction passes through the formation of a thiocarbonate intermediate, and that the subsequent CO2 release is kinetically not difficult. Nevertheless, the protein environment has an important role to play in making the latter process thermodynamically favored. No low-barrier pathway for the product release could be obtained for the reaction of n-butylisocyanide, which is consistent with the experimental fact that n-butylisocyanide inhibits Mo–Cu CODH.
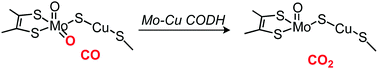


 Please wait while we load your content...
Please wait while we load your content...