Study of oxygen evolution reaction on amorphous Au13@Ni120P50 nanocluster
Abstract
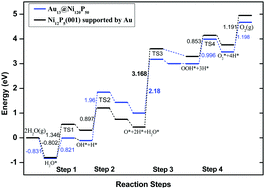
The pursuit of catalysts to promote effective water oxidization to produce oxygen has become a research subject of high priority for water splitting. Here, first-principles calculations are employed to study the water-splitting oxygen evolution reaction (OER) on ∼1.5 nm diameter Au13@Ni120P50 core–shell nanoclusters. Water splitting to produce oxygen proceeds in four intermediate reaction steps (OH*, O*, OOH* and O2). Adsorption configurations and adsorption energies for the species involved in OER on both Au13@Ni120P50 cluster and Ni12P5(001) supported by Au are presented. In addition, thermodynamic free energy diagrams and kinetic potential energy changes are systematically discussed. We show that the third intermediate reaction (O* reacting with H2O to produce OOH*) of the four elementary steps is the reaction-determining step, which accords with previous results. Also, the catalytic performance of OER for Au13@Ni120P50 is better than that for Ni12P5(001) supported by Au in terms of reactive overpotential (0.74 vs. 1.58 V) and kinetic energy barrier (2.18 vs. 3.17 eV). The optimal kinetic pathway for OER is further explored carefully for the Au13@Ni120P50 cluster. The low thermodynamic overpotential and kinetic energy barrier make Au13@Ni120P50 promising for industrial applications as a good OER electrocatalyst candidate.


 Please wait while we load your content...
Please wait while we load your content...