Doping stability of nonphotorefractive ions in stoichiometric and congruent LiNbO3
Abstract
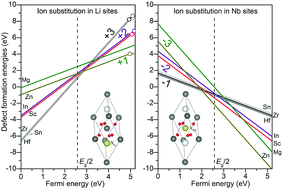
The doping stability of various nonphotorefractive ions including Mg, Zn, In, Sc, Sn, Hf, and Zr in stoichiometric, congruent, and Fe-doped LiNbO3 has been investigated by hybrid density functional theory. It is found to be energetically prefer for all the dopants to incorporate into Li sites of all the considered LiNbO3 samples at their highest charge states. The dopants with higher charge states and d electron states show lower formation energies. The introduction of intrinsic antisite NbLi and Li vacancy VLi point defects as well as extrinsic Fe dopants could promote the nonphotorefractive dopant incorporation, but did not change the relative stability order of the investigated dopants. It is found that the amount of intrinsic Nb4+/2+Li photorefractive centers is not only reduced by dopant substitution but also by the energetic suppression of the nonphotorefractive ion incorporation. Intrinsic point defects and the dopant substitution preference in NbLi or normal Li sites both play key roles in the relative order of the doping threshold concentration. Moreover, the energetics and electronic structures of the nonphotorefractive dopants and Fe codoped LiNbO3 are also investigated in this work.


 Please wait while we load your content...
Please wait while we load your content...