Reactions of SO2 and NH3 with epoxy groups on the surface of graphite oxide powder†
Abstract
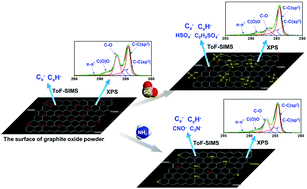
Graphite oxide powder was obtained using the modified Hummers’ method and characterized using X-ray photoelectron spectroscopy (XPS) and time-of-flight secondary ion mass spectrometry (ToF-SIMS). The XPS results indicated that the epoxy groups were the main functional groups on the graphite oxide powder surface. The graphite oxide powder was then reacted with SO2 and NH3 gases, respectively, at 25 °C. The XPS and ToF-SIMS analyses of the surface of the reacted graphite oxide powder showed that the reactions mainly occurred in the epoxy groups. Bisulfate and amine groups were formed on the surface of the graphite oxide powder after the reactions between the graphite oxide powder and SO2 and NH3 gases. This work demonstrates a new method of removing SO2 and NH3 gases using graphite oxide powder.


 Please wait while we load your content...
Please wait while we load your content...