Stability of the zwitterionic liquid butyl-methyl-imidazol-2-ylidene borane†
Abstract
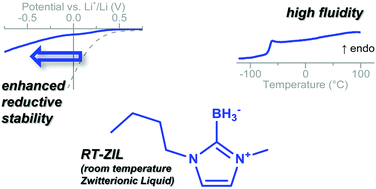
Modification of the C2 position of the standard 1-butyl-3-methyl imidazolium cation by a borohydride group leads to a zwitterionic liquid (ZIL). The resulting imidazol-2-ylidene borane ZIL is liquid at room temperature. Dynamic viscosity as well as thermal and electrochemical stability are investigated. Thermal decomposition follows a similar pathway as in comparable imidazolium ionic liquids. The surprisingly low viscosity and good reductive stability make it a promising candidate for electrochemical applications.


 Please wait while we load your content...
Please wait while we load your content...