Phase behaviours of a cationic surfactant in deep eutectic solvents: from micelles to lyotropic liquid crystals†
Abstract
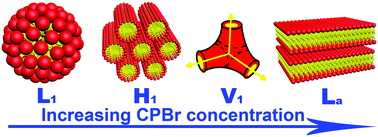
In recent years, aggregates formed in deep eutectic solvents (DESs), especially micelles, have attracted much attention. In this study, the phase behaviours of a cationic surfactant, cetylpyridinium bromide (CPBr), in two DESs, choline chloride + glycerol (ChG) and choline chloride + ethylene glycol (ChEG), were investigated in wide concentration and temperature ranges. With the help of small angle X-ray scattering, polarized optical microscopy and rheological measurements, the structures and properties of various aggregates were characterized. The micelles, hexagonal phase, bicontinuous cubic phase and lamellar phase were observed with the increase of CPBr concentration. Such rich phase behaviours were due to the large cohesive energy densities of DESs. Comparative studies in water and ethylammonium nitrate were carried out to explore how well DESs acted as self-assembly media.


 Please wait while we load your content...
Please wait while we load your content...