Ternary CBe4Au4 cluster: a 16-electron system with quasi-planar tetracoordinate carbon†
Abstract
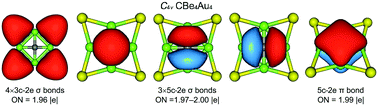
Planar hypercoordinate carbons as exotic chemical species are dominated by 18-electron counting. We report herein a 16-electron planar tetracoordinate carbon (ptC) cluster, CBe4Au4, which is quasi-planar to be exact, being composed of a C center, a square-planar Be4 ring, and four outer Au bridges. The quasi-ptC cluster is established as a global minimum via computer structural searches, located 14.6 kcal mol−1 below the nearest competitor at the CCSD(T) level. It shows thermodynamic and electronic robustness, with a low electron affinity (1.54 eV at B3LYP) and a large HOMO–LUMO gap (2.21 eV for excitation energy). Bonding analyses reveal 2π and 6σ double aromaticity, in addition to four three-center two-electron (3c-2e) Be–Au–Be σ bonds, confirming that 16-electron counting is perfect for the system. We believe that double (π and σ) aromaticity is a general concept that governs planar or quasi-planar carbons, which overrides the 18-electron rule. Competition between quasi-ptC and tetrahedral carbon (thC) isomers in the CBe4M4 (M = K, Au, H, Cl) series is also examined, which sheds crucial light on factors that govern the ptC clusters. The present findings offer opportunities for further planar and unconventional molecules.


 Please wait while we load your content...
Please wait while we load your content...