Structures of FEC-containing electrolytes and the stabilization mechanism at high voltage and elevated temperature†
Abstract
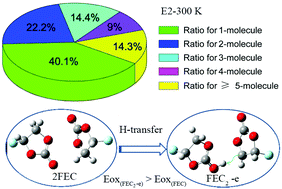
The performance of lithium-ion batteries is strongly dependent on the properties of electrolytes. Understanding of the role of electrolytes is significant for the performance improvement of batteries. In this work, classical molecular dynamic simulations were used to examine the solvation effects of ions and structures of fluoroethylene carbonate (FEC)-containing electrolytes. Density functional theory (DFT) calculations were also used to predict the oxidation stabilities of such electrolytes. The FEC-related species have higher oxidation potentials (Eox) than traditional carbonate electrolytes, indicating their good oxidation stability in high-voltage systems at elevated temperatures. The ratios of species are calculated to be used as a semi-quantitative reference for selecting the representative components of electrolytes. Then, DFT calculations on the representative components show that the oxidation potentials of coordinating species are significantly lower than those of isolated molecules when oxidation is coupled with H-transfer. Ratios of species and corresponding Eox values should be considered to accurately predict the oxidation stability of electrolytes with different solvents.


 Please wait while we load your content...
Please wait while we load your content...