Pressure-induced chemical reactions in the N2(H2)2 compound: from the N2 and H2 species to ammonia and back down into hydrazine
Abstract
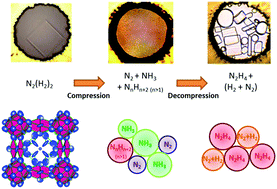
Theory predicts a very rich high pressure chemistry of hydronitrogens with the existence of many NxHy compounds. The stability of these phases under pressure is being investigated by the compression of N2–H2 mixtures of various compositions. A previous study had disclosed a eutectic-type N2–H2 phase diagram with two stoichiometric van der Waals compounds: (N2)6(H2)7 and N2(H2)2. The structure and pressure induced chemistry of the (N2)6(H2)7 compound have already been investigated. Here, we determine the structure of the N2(H2)2 compound and characterize using Raman spectroscopy measurements the chemical changes under a pressure cycle up to 60 GPa and back to ambient conditions. A N2(H2)2 single crystal was grown from a 1 : 2 N2–H2 mixture and its crystalline structure was solved using synchrotron X-ray diffraction. Similar to the (N2)6(H2)7 solid, N2(H2)2 has a remarkable host–guest structure containing N2 molecules orientationally disordered with spherical, ellipsoidal and planar shapes. Above 50 GPa, N2(H2)2 was found to undergo a chemical reaction. The reaction products were determined to be of the azane family, with NH3 as the main constituent, along with molecular nitrogen. Upon pressure decrease, the reaction products are found to react in such a way that below 10 GPa, hydrazine is the sole azane detected. Observed down to the opening of the diamond anvil cell, the formation of metastable hydrazine instead of the energetically favorable ammonia is puzzling and remains to be elucidated. That could change the current view of Jovian planets’ atmospheres in which ammonia is assumed the only stable hydronitrogen molecule.


 Please wait while we load your content...
Please wait while we load your content...